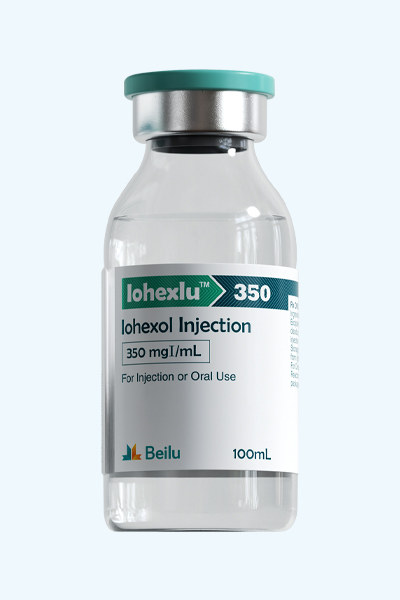
Iohexol Injection
Iohexol Injection is a non-ionic Contrast Media. Compared with a traditional ionic Contrast Media, Iohexol Injection has an excellent molecular structure and it can greatly improve user safety and significantly reduce adverse reactions. The iodinated contrast agent has excellent clinical feedback and good imaging results. In 1998, Iohexol Injectionwas launched as Beilu's 1st iodinated Contrast Media.
Product name: Iohexol Injection
Generic name: Iohexol Injection
Type: Injectable Contrast Media for CT/X-ray
Ingredients: Iohexol
Specification:
(1) 20 ml: 6 gI (2) 50 ml: 15 gI
(3) 75 ml: 22.5 gI (4) 100 ml: 30 gI
(5) 20 ml: 7 gI (6) 50 ml: 17.5 gI
(7) 100 ml: 35 gI
Administration: For injection or oral use of iodinated contrast
Adverse reactions: See the details from the package insert
Shelf life: 36 months
Indications of Iohexol Injection
The omnipaque iohexol is an X-ray contrast agent. It can be used for angiocardiography, arteriography, urography, enhanced CT examination inclduing cervical, thoracic and lumbar myelography, CT cisternography following intrathecal injection (i.e., an injection into the subarachnoid space); arthrography, endoscopic retrograde cholangiopancreatography (ERCP), herniography or fistulography, hysterosalpingography, sialography, percutaneous transhepatic cholangiography (PTC), sinography, gastroenterography, T-tube angiography, etc.
Precautions of Iohexol Injection
General precautions for the use of nonionic-monomer contrast agent:
Identify patients with high-risk factors.
Ensure patients are appropriately hydrated and, if necessary, the infusion may be maintained intravenously prior to examination until the contrast agent is cleared from the kidneys.
Avoid any nephrotoxic agent, oral gallbladder contrast agent, surgery for arterial occlusion, renal angioplasty or other major surgeries that increase the renal burden until the contrast agent is cleared.
Repeat radiographic testing is delayed until renal function returned to the pre-examination level.
To prevent lactic acidosis, the level of serum creatinine must be determined before intravascular injection of iodinated contrast agent into diabetic patients taking metformin. For patients with abnormal serum creatinine/renal function, metformin must be discontinued and the examinations with contrast agent must be postponed until after 48 hours of discontinuation. Metformin should be reused only after renal function and serum creatinine level are constant. In some emergency cases of patients with abnormal or unknown renal function, the physician must assess both advantages and disadvantages of using contrast agent and take precautions, such as discontinuation of metformin, adequate hydration of patients, testing of renal functions, and careful observation of symptoms of lactic acidosis.
There is a potential risk of transient hepatic dysfunction. Special attention should be paid to patients with serious hepatic and renal insufficiency due to significantly longer time to clear contrast agent. Hemodialysis patients may receive the examinations with contrast agents. Immediate hemodialysis is not necessary after the injection of contrast agent because there is no evidence showing that hemodialysis can protect patients with renal impairment from contrast-induced nephropathy.
Iodinated contrast agents may exacerbate symptoms of myasthenia gravis. Patients with pheochromocytoma should be treated with α-blockers to prevent hypertensive crisis in interventional procedures. Special attention should also be paid to patients with hyperthyroidism. Patients with multinodular goitre may develop hyperthyroidism after using iodinated contrast agents. The possibility of transient hypothyroidism in preterm infants after using contrast agents should be clearly recognized.
Contrast media extravasation (CME) occasionally causes localized pain or oedema, which gradually subsides without sequelae. However, inflammation and even tissue necrosis may occasionally occur. Routine treatment is to elevate the affected limb and cold-compress at local sites. Surgical decompression is required in case of compartment syndrome.
Patients should be observed for at least 30 minutes after the use of the contrast agent because most serious adverse reactions occur during this period. However, there is still the possibility of a delayed response.
After myelography, patients should rest for 1 h with 20°-raised head and chest. Patients can carefully get out of bed and walk without bending over. If patients are still in bed, keep their head and chest raised for 6 hours. Patients with low seizure thresholds should be closely monitored during this period, and outpatients should not stay alone for the first 24 hours.
Do not drive a vehicle or operate a machine within 24 hours after intraspinal injection.
ith all non-gastrointestinal drugs, this product should be visually inspected before use to check for particulate matter, discoloration and container damage.
This type of Iohexol contrast agent should be drawn into the barrel before use. Each vial is for single use only, and the unused portion should be discarded.
Beiluisa professional contrast media manufacturing companies, we provide ct contrast medium, iohexol package insert, omnipaque package insertand etc. Want to know more? Please contact us.
Send product request
Other supplier products
| Anti-Diabetic Drugs | The number of people with diabetes rose from 151 million in 2000 to 463 million in 2019; by 2045 this number will rise to 700 million. 1 in 5 of th... | |
| Contrast Media Manufacturing Company | For the purpose of medical imaging, a certain substance is introduced into the human body to change the image contrast of local tissues of the body... | |
| CT/X-Ray Contrast Media | The main component of CT/X-ray Contrast Media is iodine. The Iodinated Contrast Media is a colorless, transparent, slightly viscous liquid. At pres... | |
| Iohexol Injection | What Is Iohexol? Iohexol Injection is one of the different types of contrast media. Compared with a traditional ionic Contrast Media, Iohexol Or... | |
| Contrast Media Manufacturing Company | For the purpose of medical imaging, a certain substance is introduced into the human body to change the image contrast of local tissues of the body... |
Same products
| FSWB450-HE10 for whole blood & bedside use | Seller: Guangzhou DaJi Medical Science and Technology Co., Ltd | Computer generated filter housing with optimal characteristics Melt-blown non-woven polyester fi... | |
| Bulk Supply Chlortetracycline Premix | Seller: Hebei Shengxue Dacheng Pharmaceutical Co., Ltd. | Precautions for the use of Chlortetracycline Premix in livestock feed 1. Strict compliance: &midd... | |
| Chlortetracycline Premix Exporter | Seller: Hebei Shengxue Dacheng Pharmaceutical Co., Ltd. | Chlortetracycline Premix in livestock and poultry feed: 1. Purpose: · Promote the growth o... | |
| Chlortetracycline Premix Supplier | Seller: Hebei Shengxue Dacheng Pharmaceutical Co., Ltd. | Uses of Chlortetracycline Premix ·Livestock and poultry health: Chlortetracycline Premix i... | |
| Chlortetracycline Premix 150g200g1000g | Seller: Hebei Shengxue Dacheng Pharmaceutical Co., Ltd. | Characteristics of Chlortetracycline Premix ·Natural fermentation products: Chlortetracycl... |