Pidotimod


1. Basic Information
Product Name: Pidotimod
Aliases: Pulimo, Puleyi, Pidotimod
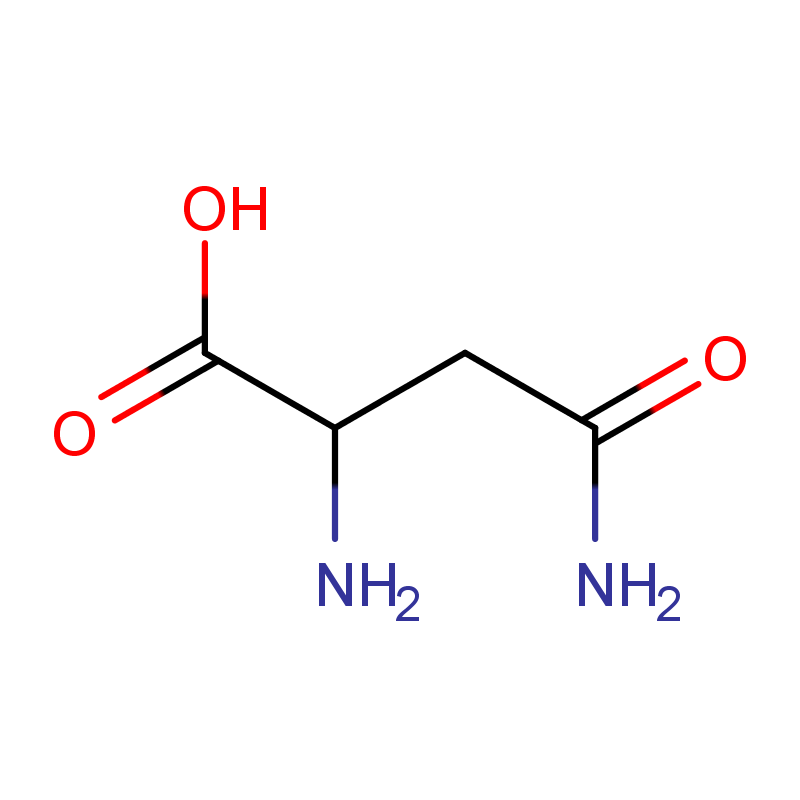
Main Chemical Component: 3-L-pyroglutamyl-thiazolidine-4-carboxylic acid
Molecular Formula: C9H12N2O4S
Molecular Weight: 244.27
2. Drug Type and Classification
Drug Type: Immunomodulator, classified as an immune enhancer.
Dosage Forms: Includes tablets (400 mg), powder for injection (400 mg), oral solution (e.g., 10 ml: 0.2 g, 10 ml: 0.4 g), and granules (e.g., 2 g: 0.4 g).
3. Efficacy and Functions
·Immune Function Enhancement: Pidotimod enhances the body's immune function, promoting both non-specific and specific immune responses.
·Infection Prevention: Used to prevent acute infections, shorten disease duration, and reduce severity.
·Adjunctive Therapy: Serves as an adjunct medication during acute infection phases and for chronic or recurrent respiratory and urinary tract infections.
4. Dosage and Administration
·Children and Adolescents: For ages 3 years and older, 0.4 g per dose, twice daily, for a course not exceeding 60 days.
·Adults: 0.8 g per dose, twice daily, with the same course duration.
5. Precautions and Contraindications
Precautions:
·Potential side effects include gastrointestinal disturbances (nausea, vomiting, diarrhea, abdominal pain), headache, dizziness, etc., generally mild.
·When taken with food, bioavailability decreases by 50%, and the time to peak plasma concentration is delayed by 2 hours. Therefore, it is recommended to take the medication apart from meals.
·In patients with renal impairment, the elimination half-life is prolonged; use with caution.
Contraindications:
·Contraindicated in individuals allergic to Pidotimod.
·Not for use in children under 3 years old.
·Pregnant women in their first trimester should use cautiously.
·Patients with a history of severe allergies should use cautiously.
·Patients with hereditary fructose intolerance or glucose-galactose malabsorption cannot use this medication.
6. Drug Metabolism and Excretion
Pidotimod is rapidly absorbed when taken orally, with a bioavailability of 37%-45% and a half-life of 4 hours.
The drug is excreted in its unchanged form (95% of the intravenously administered dose) through urine. The elimination half-life is prolonged in patients with renal impairment.
7. Controversies and Discussions
Although Pidotimod is widely used in clinical practice, large-scale clinical trial data on its efficacy and safety are insufficient.
Some experts note that while Pidotimod was introduced as an immunomodulator, subsequent large-scale studies have not confirmed its effectiveness in autoimmune diseases.
Pidotimod is an immunomodulator with functions such as enhancing immune response, preventing infections, and providing adjunctive therapy. Its usage requires attention to dosage, potential side effects, and contraindications, following the guidance of healthcare professionals. The ongoing debate over its efficacy and safety highlights the need for careful evaluation of its risks and benefits during use.
Other supplier products
|
|
Pidotimod |
1. Basic Information Product Name: Pidotimod Aliases: Pulimo, Puleyi, Pidotimod Main Chemical Component: 3-L-pyroglutamyl-thiazolidine-4-carboxylic... |
|
|
High purity Phospho-L-Tyrosine Disodium Salt |
Phospho-L-Tyrosine Disodium Salt in Biochemical Research: Standards and Assay ToolsPhosphorylation Detection Standards: Used as a reference standar... |
|
|
Hydantoin Wholesale Price |
Hydantoin in Chemical Industry Intermediates for Organic Synthesis: Hydantoin is a valuable intermediate in the synthesis of various organic compou... |
|
|
L-Aspartic acid monosodium salt monohydrate Market quotation |
The sodium component of L-Aspartic acid monosodium salt monohydrate contributes to electrolyte balance, making it a secondary ingredient in hydrati... |
|
|
Hydantoin Supply price |
Hydantoin and its derivatives can act as chelating agents. They can form stable complexes with metal ions through the nitrogen and oxygen atoms in ... |
All supplier products
Same products
|
|
Glycine Powder Exporter |
Seller: Shanghai Yifu Food Ingredients Co., Ltd |
Glycine is a non-essential amino acid naturally present in the human body and is also a substance... |
|
|
Glycine Purchase price |
Seller: Shanghai Yifu Food Ingredients Co., Ltd |
Glycine is an endogenous amino acid in the human body and a fundamental component of proteins. It... |
|
|
Glycine Partners,long-termCooperation |
Seller: Shanghai Yifu Food Ingredients Co., Ltd |
Glycine is a food-grade amino acid approved by the state. Due to its high safety, sweet taste, an... |
|
|
Glycine Marke quotation |
Seller: Shanghai Yifu Food Ingredients Co., Ltd |
Glycine has three reaction centers: amino group, carboxyl group, and methylene group. It has typi... |
|
|
Glycine Supply price |
Seller: Shanghai Yifu Food Ingredients Co., Ltd |
Physical properties of glycine1. Appearance and OdorAt room temperature, it is a white crystallin... |