Gadopentetate Dimeglumine Injection/API
Since Gadopentetate DimeglumineInjection was launched in 1992 by BeiluPharma in China, it was highly recommended and accepted by the radiologist, and quickly occupied the market with its excellent quality and reasonable price. Up to now, linear gadolinium has been safely used for nearly 30 years, with excellent enhancement effects and few side effects. Beilu also produces gadobutrol injection with high quality and reasonable price, contact us for more info!
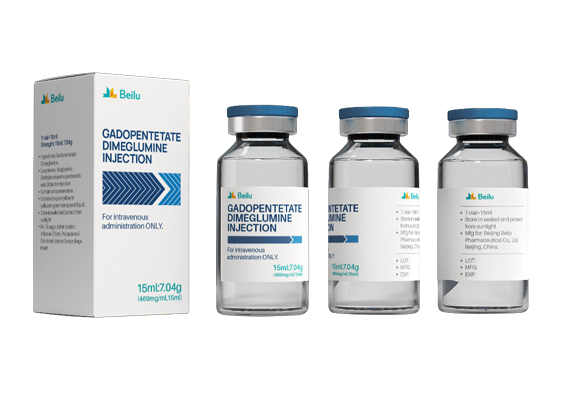
Product name: Gadopentetate Dimeglumine Injection
Generic name: Gadopentetate Dimeglumine Injection
Type: Injectable Contrast Media for MRI, Linear Contrast Media
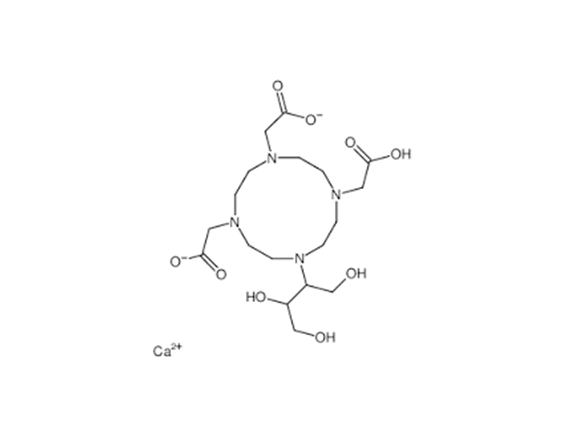
Ingredients: Gadopentetate Dimeglumine
Specification: 10ml:4.69 g; 12ml:5.63 g; 15ml:7.04 g; 20ml:9.38 g
Posology and method of administration: Intravenous injection
Adverse reactions: See the details from the package insert
Shelf life: 36 months
MRI Contrast Media-Gadopentetate Dimeglumine Injection/API
MRI Contrast Media-Gadopentetate Dimeglumine Injection/API
Indication of Gadopentetate Dimeglumine Injection
Magnetic resonance imaging of the central nervous system (brain and spinal cord), abdomen, chest, pelvis, limbs and other human organs and tissues.
Precautions of Gadopentetate Dimeglumine Injection
Current evidence shows that after repeated use of GBCAs, trace amounts of gadolinium can remain in the brain and other body tissues. Research reports have shown that multiple uses of GBCAs can increase the intensity of brain signals, especially in the dentate nucleus and globus pallidus. Currently, there are more reports about linear GBCAs and fewer reports about macrocyclic GBCAs. Animal experiments have shown that the amount of gadolinium deposited after repeated use of linear GBCAs is higher than that of repeated use of macrocyclic.
The clinical significance of brain gadolinium deposition is unclear.
In order to minimize the potential risks associated with gadolinium deposition in the brain, it must be used in strict accordance with the indications and approved doses. It is recommended to use the lowest approved dose that meets the requirement of diagnosis and perform a careful benefit-risk assessment and patient informed communication before repeated administration.
There are many contrast media manufacturing companies, but we are one of the best choices for you.
在线联系供应商
Other supplier products
| CT/X-Ray Contrast Media | The main component of CT/X-ray Contrast Media is iodine. The Iodinated Contrast Media is a colorless, transparent, slightly viscous liquid. At pres... | |
| Contrast Media Manufacturing Company | For the purpose of medical imaging, a certain substance is introduced into the human body to change the image contrast of local tissues of the body... | |
| Ferric Ammonium Citrate Effervescent Granules | Ferric Ammonium Citrate Effervescent Granules was launched in 2004. It is an oral magnetic resonance contrast agent/media approved by NMPA in the m... | |
| Calcobutrol | CAS No.: Molecular formula: C18H32CaN4O9 Molecular weight: 488.5461 Quality standard: CP Characteristics: White or off-white powder ... | |
| Contrast Media Manufacturing Company | For the purpose of medical imaging, a certain substance is introduced into the human body to change the image contrast of local tissues of the body... |
Same products
| Micronized polypropylene wax for injection moulding | 卖方: Syntop chemical Co.,Ltd. | The incorporation of polypropylene micronized wax into injection moulding processes delivers the ... | |
| Drum Type Mobile Mixing Station | 卖方: Yousheng Machinery Equipment Co.,Ltd | Drum Type Mobile Mixing Station Drum Type Mobile Mixing StationPortable Drum Concrete Batch Plan... | |
| Washable Cheap 13.56Mhz 213 Nfc Mini Stickers 13.56 Mhz RFID Label Sticker Tag HF/UHF Tags Dry Inlay | 卖方: XIUCHENG RFID | ize:On request Material:PET, PVC,paper or customized Frequency:UHF/HF Printing:Thermal transfe... | |
| Micronized wax used for industrial paint processing | 卖方: Syntop chemical Co.,Ltd. | Micronized wax is a vital functional additive in industrial paint processing, with primary functi... | |
| Plant Growth Regulator Manufacturer | 卖方: HEBEI LAIKE BIOTECH CO.LTD | Plant Growth Regulator Manufacturer Plant Growth Regulator Manufacturer - Laike Biotech spec... |