Stapled Peptides
Peptides that have been "hydrocarbon stapled" are peptides that have been chemically braced with an all-hydrocarbon staple at a specified position to keep them in their bioactive alpha-helical shape. To address the shortcomings of two major kinds of therapeutic agents (small molecules and protein biologics) in focusing on intracellular protein-protein interactions, the concept of peptide stapling was developed. Small molecules only interact with proteins that have a particular surface characteristic, and the majority of protein biologics do not enter cells. Cells are easily penetrated by stapled peptidesbecause they are locked into a stable -helical structure, which is the most prevalent component of protein secondary structures. Stapled peptides, a rapidly developing class of next-generation pharmaceuticals, are anticipated to combine the three-dimensionality and adaptable target identification capabilities of biologics with the synthetic manipulability and cell penetration of small molecules. The company you should use to manufacture your stapled peptide needs is CPC Scientific because it has extensively developed stapled peptide architectures. You are welcome to contact our technical consultants at any moment to discuss your demands for structural design.
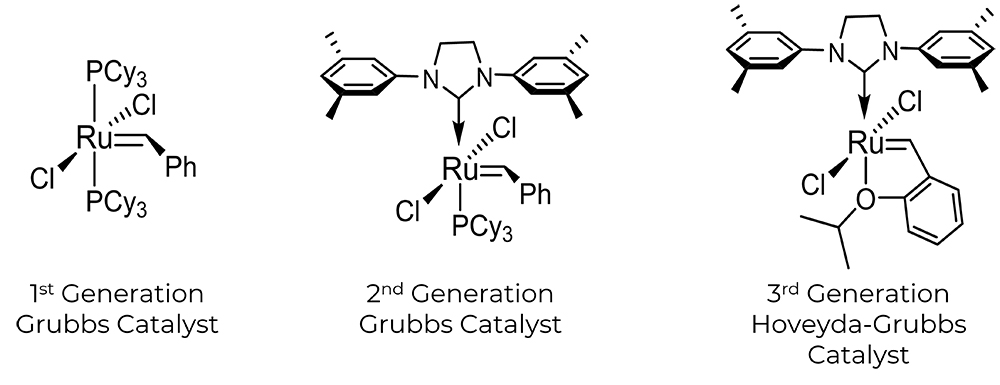
Stapled Peptide Chemistry
The first-generation Grubbs catalyst is a relatively stable ruthenium complex utilized for olefin metathesis in peptides. It has tricyclohexylphosphine (PCy3) ligands and apical-positioned carbene carbon. The creation of a second-generation Grubbs catalyst that is more thermally stable is the result of later research (middle). A third-generation catalyst, also known as the Hoveyda-Grubbs catalyst (right), substitutes benzylidene ligands with chelating ortho-isopropoxy groups on the benzene ring for N-heterocyclic carbene ligands.
lone peptide reaction stapled. A single staple peptidecan be produced by the Grubbs reaction when two alpha-4-n-pentenylalanine (S5) residues are incorporated into a peptide strand. This process is known as ring-closure metathesis. The staple type is referred to as an i, i + 4 when n = 3 (i.e., when there are 3 amino acids between the S5 residues).
In order to insert hydrocarbon building blocks into peptides during olefin metathesis, Grubbs catalysts are frequently utilized. The development of macro-cyclic bridges (conformational constraint) and,-di-substitution (helix nucleation by -methylation) are two different conformational techniques that are used to generate and stabilize a -helical shape.
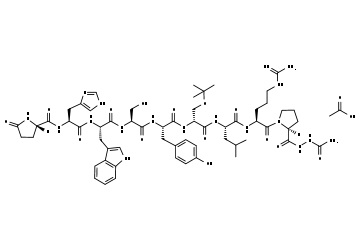
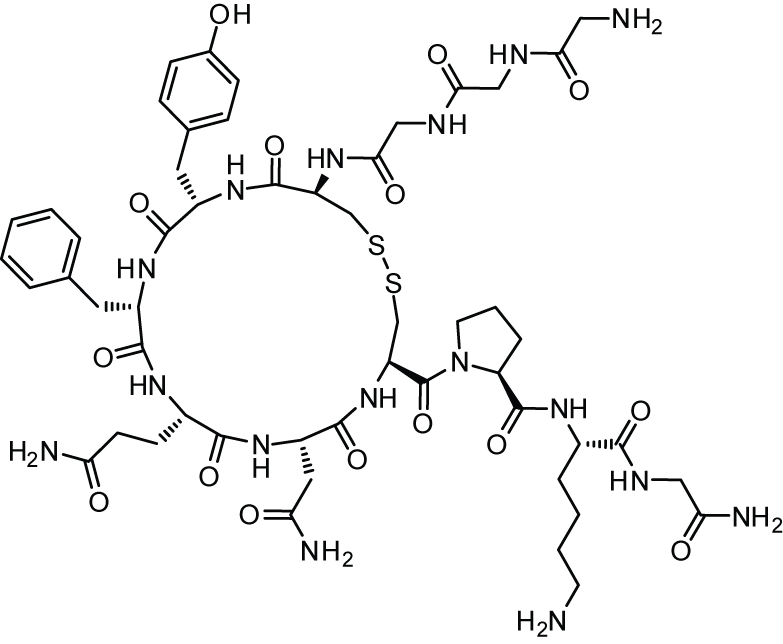
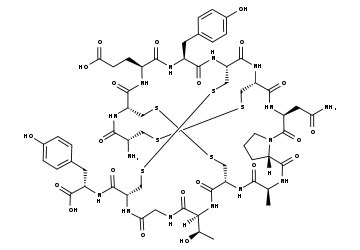
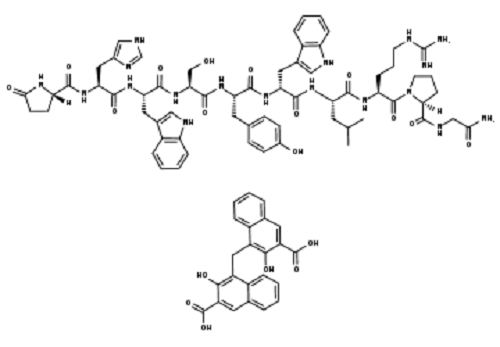
Phillips and coworkers created the sequence Ac-His-S5-Ile-Leu-His-S5-Leu-Leu-Gln-Asp-Ser-NH2 (olefin bond between S5 and S5) in a stapled peptide made by CPC Scientific, where S5 is alpha-4-n-pentenylalanine before olefin metathesis (figure 2). When the peptide is completely protected and linked to the resin as Ac-His(Trt)-S5-Ile-Leu-His(Trt)-S5-Leu-Leu-Gln(Trt)-Asp(otBu)-Ser(tBu)-Rink Amide, the Grubbs reaction is carried out. MBHA Resin. The peptide resin was mixed with 20 mL of a bis(tricyclohexylphosphine)benzylidine ruthenium (IV) dichloride (1 mg/mL) solution in DCE. The reaction took place for two hours at 50 °C. In this illustration, the peptide is bound to the resin as the stapling process (also known as Grubbs Metathesis) is being carried out. In the image, two distinct kinds of hydrocarbon staples are used to illustrate how a stabilized -helix can be formed in a peptide. I and i+4 and i, i+7 would correspond to roughly one and two revolutions of the helix, respectively. Please consult the Cahn-Ingold-Prelog priority guidelines for naming stereoisomers for details on the R/S descriptors depicted in the picture.
A double hydrocarbon stapled peptideswas created to mimic the binding domain of the human angiotensin-converting enzyme 2 (ACE2) receptor for SARS-CoV-2 in a collaboration between the Laboratory of Molecular Modeling & Drug Design, Lindsley F. Kimball Research Institute, New York Blood Center, and CPC Scientific. To cover the 30-amino-acid long binding domain of ACE2, a double staple motif of i + 7 and i + 4 was necessary.
Single Staple Configurations
Grubb’s ring-closure metathesis may result in two types of stapled peptides, i, i + 4 and i, i + 7.
Stapled Peptides in Drug Design
High amounts of -helical content are conferred by the addition of a hydrocarbon staple, which causes:
A higher target affinity of 5 to 5,000 times
Cell penetration by endocytic vesicle trafficking and increased proteolytic resistance
selecting extracellular or intracellular proteins as a target
protein-protein interactions being hampered
Non-immunogenicity
Pharmacokinetics that work and in vivo stability
Protein Targets
Targeting of many proteins involved in diseases like cancer, diabetes, HIV, and atherosclerosis has been researched using stapled peptides. Among these proteins are:
B-cell lymphoma 2 (Bcl-2)
B-cell lymphoma-extra large (Bcl-xL)
Bcl-2-associated X protein (Bax)
Induced myeloid leukemia cell differentiation(Mcl-1)
Glucokinase (GK)
Murine double minute 2 (Mdm2)
Notch/CSL
HIV-1 capsid and HIV-1 gp41
ATP-binding cassette transporter (ABCA1)
Estrogen receptor
4 Panel Stapled W Protein
Figure 5. i, i + 4 Stapled peptide (Ac-His-S5-Ile-Leu-His-S5-Leu-Leu-Gln-Asp-Ser-NH2) bound to estrogen receptor. Stapling organizes non-adjacent leucine side chains (Leu-4 and Leu-8) project from the same side into the hydrophobic pocket of estrogen protein.[1]
Modifications of Stapled Peptides
The two types of stapled peptide modifications are fluorescent label and affinity tag, respectively. Fluorescein and biotin are two of the most prevalent moieties added to the N-terminus of stapled peptides, and they can be employed for biophysical characterisation and target interaction studies as well as studies of intracellular absorption. To separate the alteration from the peptide's core, a flexible molecular spacer is typically preferred.
N-acetylation
Linker attachment (β-alanine, mini-PEG, etc.)
Fluorescent labeling (FITC, 5-FAM, etc.)
Template-Assisted Β-Sheet Stapled Peptide
Peptide stapling can also be used to reproduce other secondary protein structures, such as -sheets. In the example below, a template-assisted olefin metathesis reaction using a third-generation Hoveyda-Grubbs catalyst converts terminal olefins into internal olefins. Due to stability of the anti-parallel -sheet configuration created by the six hydrogen bonds, only one conformer is visible.[3]
Hao Stapled Pepitde
Stapled Peptides by Click Chemistry
It is possible to easily synthesize triazole-stapled peptides thanks to the high efficiency and benign circumstances of the "click" reaction (Copper-catalyzed Huisgen 1,3-dipolar cycloaddition reaction) and the availability of the unnatural amino acids that are required. To make single triazole-stapled peptides, for instance, L-Nle (N3) and D-Pra (D-propargylalanine) can be combined and substituted at the i and i+4 locations, respectively.
New Click Stapled 1000sm
References
Phillips, Chris, et al. Journal of the American Chemical Society 133.25 (2011): 9696-9699 (PDB: 2YJA).
Curreli, Francesca, Sofia MB Victor, Shahad Ahmed, Aleksandra Drelich, Xiaohe Tong, Chien-Te K. Tseng, Christopher D. Hillyer, and Asim K. Debnath. Mbio 11, no. 6 (2020): e02451-20.
Gothard, Chris. Unpublished work in the Nowick Group (2005).
For more informationabout stapled peptide synthesis, please feel free to contact us!
There are many peptide api manufacturers, but we are one of the best choices for you.
在线联系供应商
Other supplier products
| Goserelin Acetate | Molecular Weight: 1329.4762 Goserelin Acetate Produced in: Hangzhou Goserelin Acetate Application Suppress production of the sex hormones As a pept... | |
| Terlipressin Acetate | TerlipressinSequence: H-Gly-Gly-Gly-Cys-Tyr-Phe-Gln-Asn-Cys-Pro-Lys-Gly-NH2 (disulfide bridge) Molecular Weight: inj terlipressinProduced in... | |
| Linaclotide | Linaclotide Sequence: [9-L-tyrosine]heat-stable enterotoxin (Escherichia coli)-(6-19)-peptide Molecular weight: Linaclotide Produced in: ... | |
| Triptorelin Pamoate | Triptorelin pamoate for breast cancerSequence: 5-oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L-tyrosyl-D-tryptophyl-L-leucyl-L-arginyl-L-prolylgl... | |
| Generic Peptides | In addition, CPC specializes in the production of lanreotide therapyon an industrial scale, following the CGMP's (Current Good Manufacturing Practi... |
Same products
| Trimellitic anhydride 97% | 卖方: Yufeng International Group Co., Ltd | Trimellitic anhydrideis a 2-benzofuran compound having oxo groups at the 1- and 3-positions and a... | |
| Isobutyric Anhydride CAS 97-72-3 | 卖方: Yufeng International Group Co., Ltd | Product Name: Isobutyric Anhydride CAS No.: 97-72-3 Purity: 99% Molecular Formula: C6H10O3 Mo... | |
| Isobutyric Acid CAS 79-31-2 | 卖方: Yufeng International Group Co., Ltd | Product Name:ISOBUTYRIC ACID Synonyms: 2-Methylpropanoic acid 79-31-2 Isobutanoic acid 2-Met... | |
| 2-Amino-2-methyl-1-propanol(AMP)CAS:124-68-5 | 卖方: Yufeng International Group Co., Ltd | AtYufeng, a trusted 2-Amino-2-methyl-1-propanol Factory & Supplier, we prioritize quality and... | |
| Dimethyl sulfoxide (DMSO) CAS: 67-68-5 | 卖方: Yufeng International Group Co., Ltd | Yufengis one of the leading dimethyl sulfoxide suppliers and also a professional such manufacture... |