Gadopentetate Dimeglumine Injection/API
Founded in 1992, BeijingBeiluPharmaceuticalsCompanyLimitedis mainly engaged in the research & development, production and sales of pharmaceutical products. It is high-tech innovation company with core competence in the Contrast Media domestic. It has around 650 employees with 0.82 Bn/¥ revenue in 2019. Beilu provides complete Contrast Media products Include ct contrast medium, mri contrast medium, gadopentetate dimeglumine,etc, and it is the leading manufacturer in the Contrast Media field in China. It is one of the 1st batches of listed enterprises in GEM of SZSE (Ticker: 300016.SZ) in China.
Since Gadopentetate Dimeglumine Injection was launched in 1992 by Beilu Pharma in China, it was highly recommended and accepted by the radiologist, and quickly occupied the market with its excellent quality and reasonable price. Up to now, linear gadolinium has been safely used for nearly 30 years, with excellent enhancement effects and few side effects.
Product name: Gadopentetate Dimeglumine Injection
Generic name: Gadopentetate Dimeglumine Injection
Type: Injectable magnevist contrast Media for MRI, Linear Contrast Media
No.1 market share in China
Ingredients: Gadopentetate Dimeglumine
Specification: 10ml:4.69 g; 12ml:5.63 g; 15ml:7.04 g; 20ml:9.38 g
Posology and method of administration: Intravenous injection
Adverse reactions: See the details from the package insert
Shelf life: 36 months
Indication of Gadopentetate Dimegumine Injection
Magnetic resonance imaging of the central nervous system (brain and spinal cord), abdomen, chest, pelvis, limbs and other human organs and tissues.
Precautions of Gadopentetate Dimegumine Injection
1. Use with caution in patients with severe kidney damage, epilepsy, hypotension, asthma and other allergic respiratory diseases and those with allergic tendencies.
2. Take care to avoid the extravasation of the gadopentetic acid liquid during injection to prevent tissue pain.
3. The serum iron and bilirubin levels of some patients will increase slightly after medication, but they are asymptomatic and can return to normal within 24 hours.
4. Pregnant women and breastfeeding women should use it with caution. Animal experiments show that a small amount of medicine liquid enters the milk.
5. The effective enhancement time of magnevist contrast agent / magnevist contrast dye is 45 minutes,and the magnevist price is little high. MRI examination should be performed immediately after intravenous injection.
6. The remaining medicine liquid after one examination should not be used again.
7. When applying this type of MRI contrast media, follow the relevant safety regulations in magnetic resonance imaging.
8. GBCAs should be used with caution. When plain scan MRI cannot obtain the corresponding vital diagnostic information, GBCAs can be used, and the lowest approved dose is used as much as possible.
9. Gadolinium deposition
Current evidence shows that after repeated use of GBCAs, trace amounts of gadolinium can remain in the brain and other body tissues. Research reports have shown that multiple uses of GBCAs can increase the intensity of brain signals, especially in the dentate nucleus and globus pallidus. Currently, there are more reports about linear GBCAs and fewer reports about macrocyclic GBCAs. Animal experiments have shown that the amount of gadolinium deposited after repeated use of linear GBCAs is higher than that of repeated use of macrocyclic.
The clinical significance of brain gadolinium deposition is unclear.
In order to minimize the potential risks associated with gadolinium deposition in the brain, it must be used in strict accordance with the indications and approved doses. It is recommended to use the lowest approved dose that meets the requirement of diagnosis and perform a careful benefit-risk assessment and patient informed communication before repeated administration.
在线联系供应商
Other supplier products
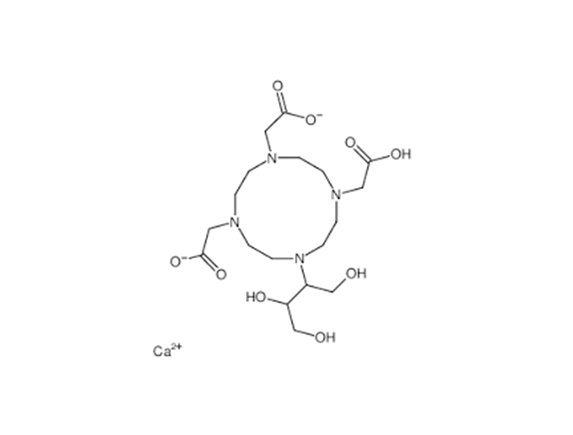
| Calcobutrol | Calcobutrol CAS No.: Molecular formula: C18H32CaN4O9 Molecular weight: 488.5461 Quality standard: CP Characteristics: White or off-... | |
| Glimepiride Tablets | Glimepiride 1 Tabletis a sulfonylurea hypoglycemic drug that can be used in combination with insulin. In 2001, glimepiride 2 tablet(Dibei), as the ... | |
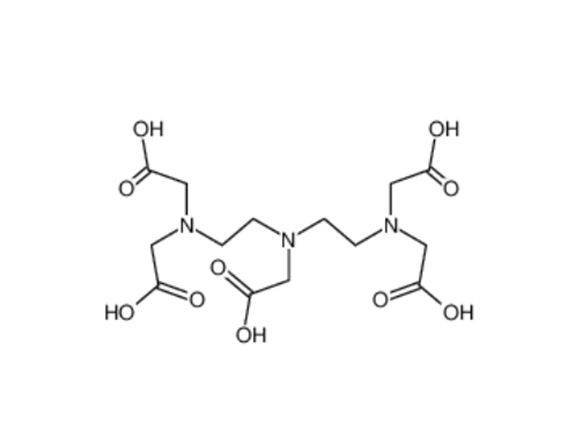
| Diethylenetriaminepentaacetic acid (DTPA) | DTPA Diethylenetriaminepentaacetic Acid(DTPA) CAS No.: 67-43-6 Molecular formula: C₁₄H₂₃N₃O₁₀ Molecular weight: 393.35 Quality standard... | |
| CT/X-Ray Contrast Media | The main component of CT/X-ray Contrast Media is iodine. The Iodinated Contrast Media is a colorless, transparent, slightly viscous liquid. At pres... | |
| Iodixanol Injection | Founded in 1992, BeijingBeiluPharmaceuticalsCompanyLimitedis mainly engaged in the research & development, production and sales of pharmaceutic... |
Same products
| FSWB450-HE10 for whole blood & bedside use | 卖方: Guangzhou DaJi Medical Science and Technology Co., Ltd | Computer generated filter housing with optimal characteristics Melt-blown non-woven polyester fi... | |
| Chlortetracycline Premix | 卖方: Hebei Shengxue Dacheng Pharmaceutical Co., Ltd. | Precautions for the use of Chlortetracycline Premix in livestock feed 1. Strict compliance: &midd... | |
| Chlortetracycline Premix | 卖方: Hebei Shengxue Dacheng Pharmaceutical Co., Ltd. | Chlortetracycline Premix in livestock and poultry feed: 1. Purpose: · Promote the growth o... | |
| Chlortetracycline Premix | 卖方: Hebei Shengxue Dacheng Pharmaceutical Co., Ltd. | Uses of Chlortetracycline Premix ·Livestock and poultry health: Chlortetracycline Premix i... | |
| Chlortetracycline Premix | 卖方: Hebei Shengxue Dacheng Pharmaceutical Co., Ltd. | Characteristics of Chlortetracycline Premix ·Natural fermentation products: Chlortetracycl... |