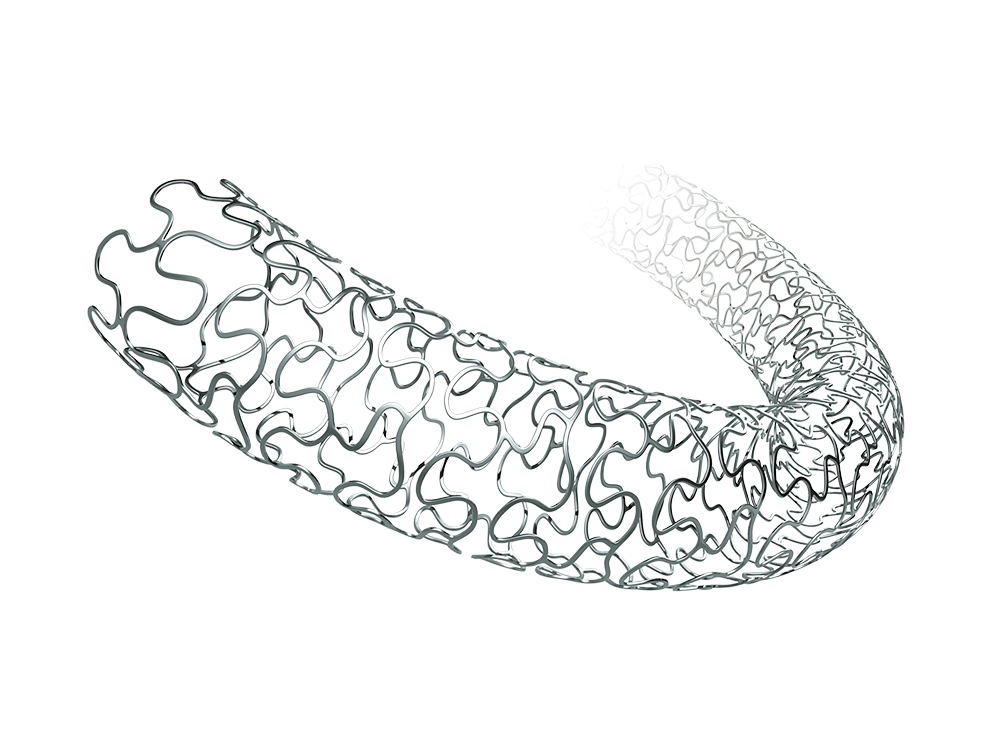
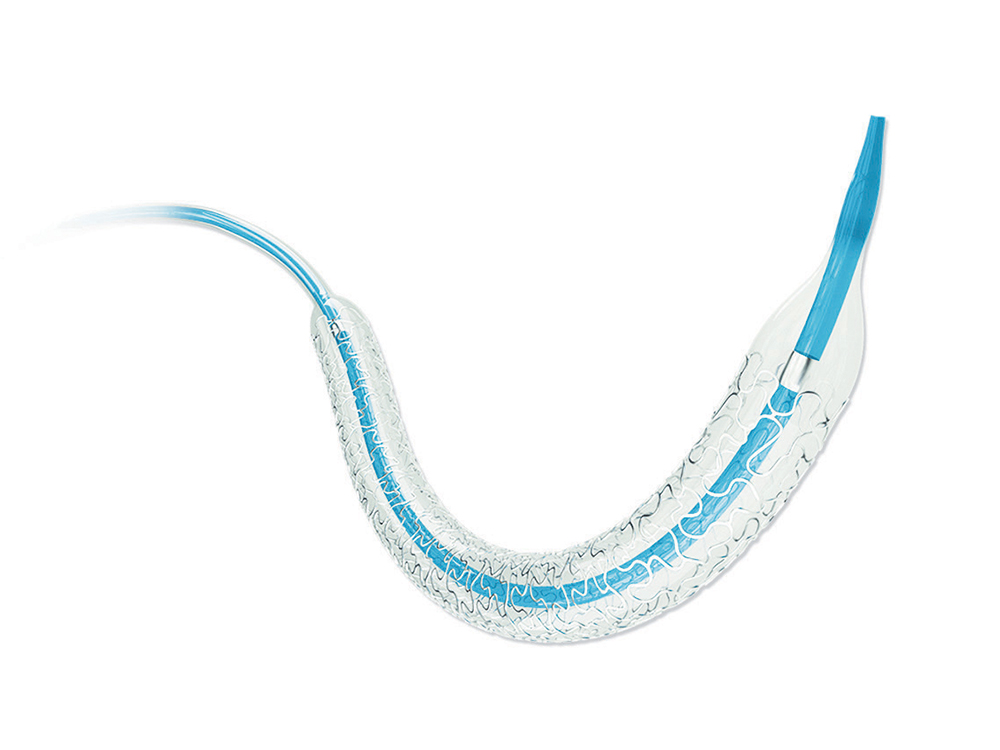
Nano+™ Polymer-free Sirolimus-eluting Coronary Stent System
Nano+™ Polymer Free Drug Eluting StentsSystem Details
Product Description
- Nanoporous cavities on strut abluminal surface functioning as drug carrier to ensure the firm adhesion.
- Vacuum drug coating technology ensures complete drug dosage and coating stability.
- Post-treatment after drug loading helps with sustained drug release.
- Soft tapered tip provides smooth transition from guidewire to balloon catheter and excellent crossability.
- Short shoulder design at both ends of the balloon ensures the accurate dilation to the lesion, and reduces the damage to normal vessel next to the lesion.
- Patented hydrophilic coating on distal shaft provides outstanding pushability.
- Polymer-free design significantly reduces the inflammatory response after stent implantation to guarantee the long-term safety.
- Polymer-free design facilitates earlier endothelialization which significantly shorten the DAPT time.
- The rough strut surface helps with earlier and easier endothelialization to reduce the risk of thrombosis.
DES Efficacy [1]
BMS Safety [2]
Nano+™ OCT Study
An OCT Study in Europe Shows Nano+™ A Great Combination of DES Efficacy and BMS Safety
Prouduct Features
- Polymer-free DES
- Nanoporous drug carrier on abluminal surface
- Balanced strut thickness
- High radial force
- Sine wave + 3-3-3 link
- Double helix structure
- Large open cell
- Precise positioning markers
- Effective drug of Sirolimus
- Vacuum drug coating technology
- 90-day drug releasing period
- New delivery system
Prouduct Benefits
- Significantly shorten DAPT time
- Earlier & better endothelialization
- Highly reduced risk of thrombosis
- Great deliverability
- Easy positioning and placement
- Optimal tissue scaffolding
- Excellent side branch access
- Scientific drug releasing period
Prouduct Ranges
- Ø 2.5 - 4.0 mm
- 12 - 36 mm multi stent lengths
Clinical Studies
An OCT Study in Europe Shows Nano+™ A Great Combination of DES Efficacy and BMS Safety [3].
In a prospective, multicentre, single-arm, open-label study, OCT examinations
were performed at three months in 45 patients (47 lesions). 23 patients with 24 lesions
had serial coronary angiography and OCT assessment at three and six months.
OCT Analyses
Coverage status of the individual stents/patients at two time points (three and six months).
Serial OCT showed almost complete vascular healing at six months, even when coverage was insufficient at three months. This suggests an adequate safety and efficacy profile of the device at that point in time.
Specifications
|
Technical Parameters |
|
|
Stent Material |
316L Stainless Steel |
|
Strut Design |
Nanoporous drug carrier on abluminal surface |
|
Metal to Artery Ratio |
15% |
|
Recoil |
< 2% |
|
Shortening |
< 1% |
|
Crossing Profile |
|
Ordering Information
|
Stent Diameter |
Stent Length (mm) |
|||||||
|
(mm) |
12 |
15 |
18 |
21 |
24 |
29 |
33 |
36 |
|
LPRPS2512 |
LPRPS2515 |
LPRPS2518 |
LPRPS2521 |
LPRPS2524 |
LPRPS2529 |
LPRPS2533 |
- |
|
|
LPRPS2712 |
LPRPS2715 |
LPRPS2718 |
LPRPS2721 |
LPRPS2724 |
LPRPS2729 |
LPRPS2733 |
LPRPS2736 |
|
|
3 |
LPRPS3012 |
LPRPS3015 |
LPRPS3018 |
LPRPS3021 |
LPRPS3024 |
LPRPS3029 |
LPRPS3033 |
LPRPS3036 |
|
LPRPS3512 |
LPRPS3515 |
LPRPS3518 |
LPRPS3521 |
LPRPS3524 |
LPRPS3529 |
LPRPS3533 |
LPRPS3536 |
|
|
4 |
LPRPS4012 |
LPRPS4015 |
LPRPS4018 |
LPRPS4021 |
LPRPS4024 |
LPRPS4029 |
LPRPS4033 |
LPRPS4036 |
Provide Comprehensive Solution For
Global Patients
Fill out the form to leave inquiries. We would get back to you as soon as possible.
As one of professional medical device manufacturers in China, we have been engaged in manufacturing medical device for many years. Thus, we have confidence to benefit the people in needs through our products.
在线联系供应商
Other supplier products
| PC-60FW Fingertip Pulse Oximeter | Artifact removal and anti-motion Splash proof and drop resistant Accurate and sensitive portable finger pulse oximeterFeatures OLED displ... | |
| Single Use Disposable Trocar and Veress Needle | 45° Sleeve Tip Bevel Navigate Thread Sealing Better Application: Lepu Medical disposable trocarare suitable for establishing the wo... | |
| Partner®Sirolimus-eluting Coronary Stent System | Partner® Sirolimus Eluting Coronary StentSystem Partner® Sirolimus-eluting Coronary Stent System details Product Features Easy positi... | |
| Sertraline HCl | Sertraline HCl Item/Code CAS No. Molecular Structure Therapeutic Area Certification/Stage API 79617-99... | |
| Disposable Circular Stapler | Disposable Circular Stapler for Hemorrhoids • Super-thin anvil, more convenient for procedure. • Bigger inner cutting diameter, minim... |
Same products
| FSWB450-HE10 for whole blood & bedside use | 卖方: Guangzhou DaJi Medical Science and Technology Co., Ltd | Computer generated filter housing with optimal characteristics Melt-blown non-woven polyester fi... | |
| Chlortetracycline Premix | 卖方: Hebei Shengxue Dacheng Pharmaceutical Co., Ltd. | Precautions for the use of Chlortetracycline Premix in livestock feed 1. Strict compliance: &midd... | |
| Chlortetracycline Premix | 卖方: Hebei Shengxue Dacheng Pharmaceutical Co., Ltd. | Chlortetracycline Premix in livestock and poultry feed: 1. Purpose: · Promote the growth o... | |
| Chlortetracycline Premix | 卖方: Hebei Shengxue Dacheng Pharmaceutical Co., Ltd. | Uses of Chlortetracycline Premix ·Livestock and poultry health: Chlortetracycline Premix i... | |
| Chlortetracycline Premix | 卖方: Hebei Shengxue Dacheng Pharmaceutical Co., Ltd. | Characteristics of Chlortetracycline Premix ·Natural fermentation products: Chlortetracycl... |