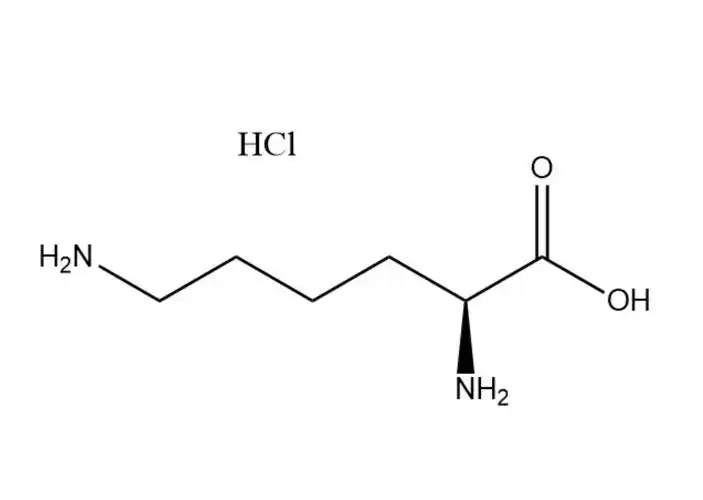
DL-methionine Partners, long-term Cooperation
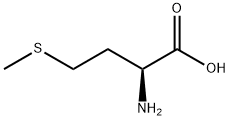
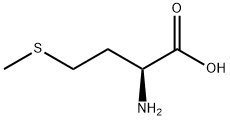
The methylthio group is the most distinctive functional group in DL-methionine and can participate in substitution and addition reactions. Alkylation substitution reaction: The sulfur atom in the methylthio group has a lone pair of electrons, making it a nucleophilic site that can react with alkyl halides (e.g., methyl iodide) to form sulfonium salts. This reaction is the basis for the synthesis of methionine derivatives with enhanced biological activity. Cleavage reaction: Under the action of strong nucleophiles (e.g., sodium hydroxide in the presence of hydrogen peroxide) or enzymes (e.g., methionine lyase), the C-S bond in the methylthio group can be cleaved, producing homocysteine and methanethiol. In biological systems, this cleavage reaction is catalyzed by specific enzymes and is a key step in methionine metabolism.
Other supplier products
|
|
L-Cysteine hydrochloride monohydrate Wholesale Price |
Handling and Storage Practices1. Sealing After UseImmediate Re-Sealing: After opening, remove the required amount quickly and re-seal the container... |
|
|
High quality L-Cysteine hydrochloride monohydrate |
The storage methods for L-Cysteine hydrochloride monohydrate are crucial to maintain its stability and functionality, primarily due to its hygrosco... |
|
|
L-Glutamine Manufacturer |
L-Glutamine is a conditionally essential amino acid with important roles in the body. Here's a detailed introduction:
1. Chemical structure and na... |
|
|
DL-Alanine Powder Price |
The original commercial packaging of DL-Alanine is designed for its stability:
Unopened products: Keep in the original sealed bag/bucket (usually... |
|
|
Wholesale DL-methionine powder |
DL-Methionine is the most widely used and largest-volume amino acid feed additive globally, playing an irreplaceable role in improving the nutritio... |
All supplier products
Same products