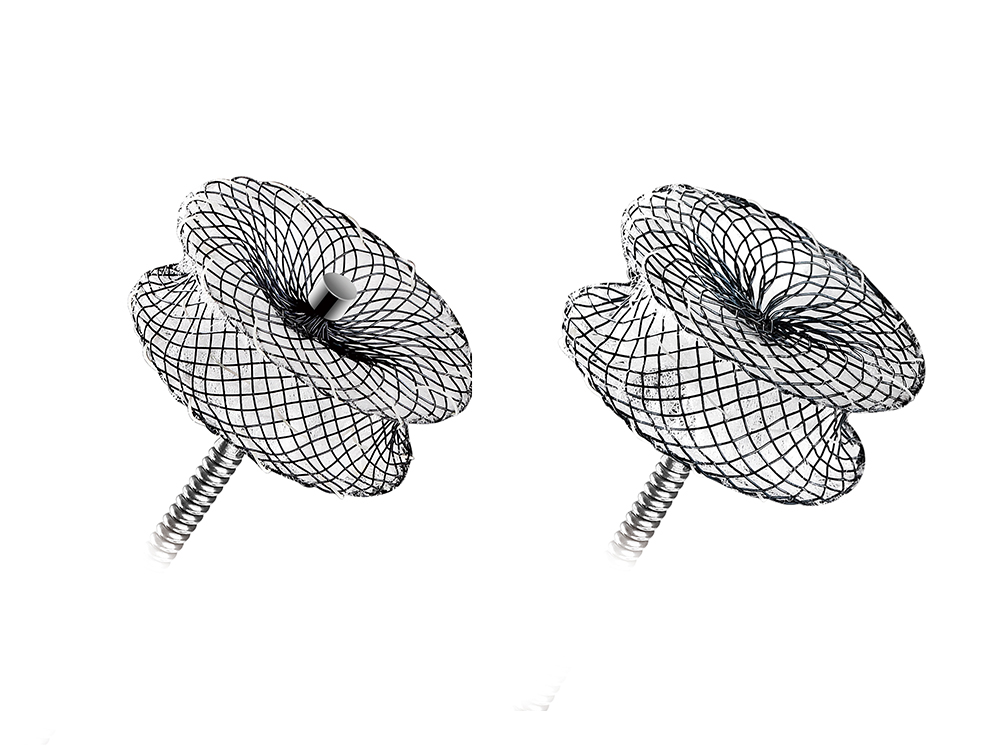
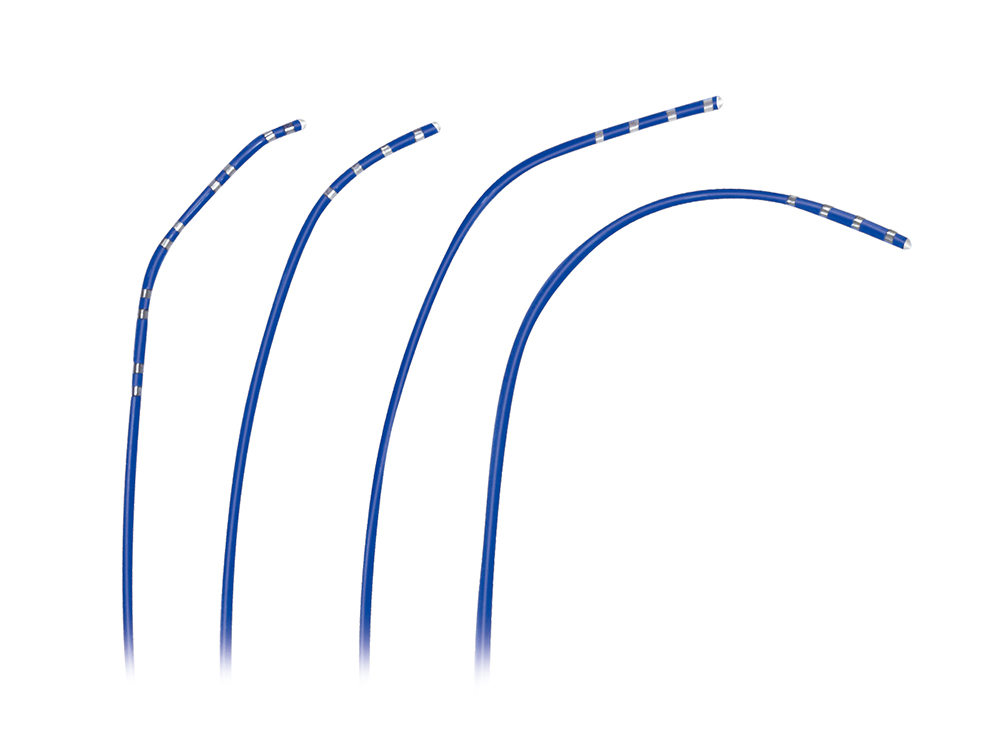
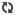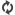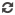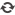MemoPart™ Ventricular Septal Defect (VSD) Occluder
MemoPart™
Fit to the clinic, long-term safety
MemoPart™ VSD OccluderDetail
Product Description
MemoPart™ Ventricular Septal Defect (VSD) Occluder is a self-expanding double-disc nitinol mesh occlusion device.The 2 discs are connected by a short waist which corresponds to the defect size. Polyester fabric is securely sewnto each disc to secure the occlusion. The device is visible under X-ray.
Indications for Use
Membranous VSD Occluder
The MemoPartTM Membranous VSD Occluder is used for minimally invasive transcatheter closure of perimembranous ventricular septal defects.
Muscular VSD Occluder
The MemoPartTM Muscular VSD Occluder is indicated for use in patients with a complex ventricular septal defect (VSD) of significant size to warrant closure (large volume left-to-right shunt, pulmonary hypertension,and/or clinical symptoms ofcongestive heart failure) who are considered to be at high risk for standard transatrial or transarterial surgical closure based on anatomical conditions and/or based on overall medical condition.
Ordering Information
MuscularVentricular Septal Defect (VSD)Occluder
|
Catalogue No |
Device Size |
A Waist Diameter (mm) |
BLV DiscDiameter(mm) |
C RV Disc Diameter (mm) |
H Waist Length (mm) |
|
SQFDQ- Ⅰ a04 |
4 |
4 |
8 |
8 |
|
|
SQFDQ- Ⅰ a05 |
5 |
5 |
9 |
9 |
|
|
SQFDQ- Ⅰ a06 |
6 |
6 |
10 |
10 |
|
|
SQFDQ- Ⅰ a07 |
7 |
7 |
11 |
11 |
|
|
SQFDQ- Ⅰ a08 |
8 |
8 |
12 |
12 |
|
|
SQFDQ- Ⅰ a09 |
9 |
9 |
13 |
13 |
|
|
SQFDQ- Ⅰ a10 |
10 |
10 |
14 |
14 |
|
|
SQFDQ- Ⅰ a12 |
12 |
12 |
16 |
16 |
|
|
SQFDQ- Ⅰ a14 |
14 |
14 |
18 |
18 |
|
|
SQFDQ- Ⅰ a16 |
16 |
16 |
20 |
20 |
|
|
SQFDQ- Ⅰ a18 |
18 |
18 |
22 |
22 |
|
|
SQFDQ- Ⅰ b04 |
4 |
4 |
10 |
8 |
|
|
SQFDQ- Ⅰ b05 |
5 |
5 |
11 |
9 |
|
|
SQFDQ- Ⅰ b06 |
6 |
6 |
12 |
10 |
|
|
SQFDQ- Ⅰ b07 |
7 |
7 |
13 |
11 |
|
|
SQFDQ- Ⅰ b08 |
8 |
8 |
14 |
12 |
|
|
SQFDQ- Ⅰ b09 |
9 |
9 |
15 |
13 |
|
|
SQFDQ- Ⅰ b10 |
10 |
10 |
16 |
14 |
|
|
SQFDQ- Ⅰ b12 |
12 |
12 |
18 |
16 |
|
|
SQFDQ- Ⅰ b14 |
14 |
14 |
20 |
18 |
|
|
SQFDQ- Ⅰ b16 |
16 |
16 |
22 |
20 |
|
|
SQFDQ- Ⅰ b18 |
18 |
18 |
24 |
22 |
|
|
SQFDQ- Ⅰ c04 |
4 |
4 |
14 |
10 |
10 |
|
SQFDQ- Ⅰ c05 |
5 |
5 |
15 |
11 |
10 |
|
SQFDQ- Ⅰ c06 |
6 |
6 |
16 |
12 |
10 |
|
SQFDQ- Ⅰ c07 |
7 |
7 |
17 |
13 |
10 |
|
SQFDQ- Ⅰ c08 |
8 |
8 |
18 |
14 |
10 |
|
SQFDQ- Ⅰ c09 |
9 |
9 |
19 |
15 |
10 |
|
SQFDQ- Ⅰ c10 |
10 |
10 |
20 |
16 |
10 |
|
SQFDQ- Ⅰ c12 |
12 |
12 |
22 |
18 |
10 |
|
SQFDQ- Ⅰ c14 |
14 |
14 |
24 |
20 |
10 |
|
SQFDQ- Ⅰ c16 |
16 |
16 |
26 |
22 |
10 |
|
SQFDQ- Ⅰ c18 |
18 |
18 |
28 |
24 |
10 |
Hubless MuscularVentricular Septal Defect (VSD)Occluder
|
Catalogue No |
Device Size |
A Waist Diameter (mm) |
BLV DiscDiameter(mm) |
C RV Disc Diameter (mm) |
H Waist Length (mm) |
|
WTSQFDQ- Ⅰ a04 |
4 |
4 |
8 |
8 |
5 |
|
WTSQFDQ- Ⅰ a05 |
5 |
5 |
9 |
9 |
5 |
|
WTSQFDQ- Ⅰ a06 |
6 |
6 |
10 |
10 |
5 |
|
WTSQFDQ- Ⅰ a07 |
7 |
7 |
11 |
11 |
5 |
|
WTSQFDQ- Ⅰ a08 |
8 |
8 |
12 |
12 |
5 |
|
WTSQFDQ- Ⅰ a09 |
9 |
9 |
13 |
13 |
5 |
|
WTSQFDQ- Ⅰ a10 |
10 |
10 |
14 |
14 |
5 |
|
WTSQFDQ- Ⅰ a12 |
12 |
12 |
16 |
16 |
5 |
|
WTSQFDQ- Ⅰ a14 |
14 |
14 |
18 |
18 |
5 |
|
WTSQFDQ- Ⅰ a16 |
16 |
16 |
20 |
20 |
5 |
|
WTSQFDQ- Ⅰ a18 |
18 |
18 |
22 |
22 |
5 |
|
WTSQFDQ- Ⅰ b04 |
4 |
4 |
10 |
8 |
7 |
|
WTSQFDQ- Ⅰ b05 |
5 |
5 |
11 |
9 |
7 |
|
WTSQFDQ- Ⅰ b06 |
6 |
6 |
12 |
10 |
7 |
|
WTSQFDQ- Ⅰ b07 |
7 |
7 |
13 |
11 |
7 |
|
WTSQFDQ- Ⅰ b08 |
8 |
8 |
14 |
12 |
7 |
|
WTSQFDQ- Ⅰ b09 |
9 |
9 |
15 |
13 |
7 |
|
WTSQFDQ- Ⅰ b10 |
10 |
10 |
16 |
14 |
7 |
|
WTSQFDQ- Ⅰ b12 |
12 |
12 |
18 |
16 |
7 |
|
WTSQFDQ- Ⅰ b14 |
14 |
14 |
20 |
18 |
7 |
|
WTSQFDQ- Ⅰ b16 |
16 |
16 |
22 |
20 |
7 |
|
WTSQFDQ- Ⅰ b18 |
18 |
18 |
24 |
22 |
7 |
|
WTSQFDQ- Ⅰ c04 |
4 |
4 |
14 |
10 |
10 |
|
WTSQFDQ- Ⅰ c05 |
5 |
5 |
15 |
11 |
10 |
|
WTSQFDQ- Ⅰ c06 |
6 |
6 |
16 |
12 |
10 |
|
WTSQFDQ- Ⅰ c07 |
7 |
7 |
17 |
13 |
10 |
|
WTSQFDQ- Ⅰ c08 |
8 |
8 |
18 |
14 |
10 |
|
WTSQFDQ- Ⅰ c09 |
9 |
9 |
19 |
15 |
10 |
|
WTSQFDQ- Ⅰ c10 |
10 |
10 |
20 |
16 |
10 |
|
WTSQFDQ- Ⅰ c12 |
12 |
12 |
22 |
18 |
10 |
|
WTSQFDQ- Ⅰ c14 |
14 |
14 |
24 |
20 |
10 |
|
WTSQFDQ- Ⅰ c16 |
16 |
16 |
26 |
22 |
10 |
|
WTSQFDQ- Ⅰ c18 |
18 |
18 |
28 |
24 |
10 |
Membranous SymmetricVentricular Septal Defect (VSD)Occluder
|
Catalogue No. |
Device Size |
AWaistDiameter(mm) |
BLV DiscDiameter(mm) |
C RV Disc Diameter (mm) |
HWaistLength(mm) |
|
SQFDQ- Ⅱ b04 |
04 |
4 |
8 |
8 |
|
|
SQFDQ- Ⅱ b05 |
05 |
5 |
9 |
9 |
|
|
SQFDQ- Ⅱ b06 |
06 |
6 |
10 |
10 |
|
|
SQFDQ- Ⅱ b07 |
07 |
7 |
11 |
11 |
|
|
SQFDQ- Ⅱ b08 |
08 |
8 |
12 |
12 |
|
|
SQFDQ- Ⅱ b09 |
09 |
9 |
13 |
13 |
|
|
SQFDQ- Ⅱ b10 |
10 |
10 |
14 |
14 |
|
|
SQFDQ- Ⅱ b12 |
12 |
12 |
16 |
15 |
|
|
SQFDQ- Ⅱ b14 |
14 |
14 |
18 |
17 |
|
|
SQFDQ- Ⅱ b16 |
16 |
16 |
22 |
20 |
|
|
SQFDQ- Ⅱ b18 |
18 |
18 |
24 |
22 |
|
|
SQFDQ- Ⅱ b20 |
20 |
20 |
26 |
24 |
Hubless Membranous SymmetricVentricular Septal Defect (VSD)Occluder
|
Catalogue No. |
Device Size |
AWaistDiameter(mm) |
BLV DiscDiameter(mm) |
C RV Disc Diameter (mm) |
HWaistLength(mm) |
|
WTSQFDQ- Ⅱ b04 |
04 |
4 |
8 |
8 |
|
|
WTSQFDQ- Ⅱ b05 |
05 |
5 |
9 |
9 |
|
|
WTSQFDQ- Ⅱ b06 |
06 |
6 |
10 |
10 |
|
|
WTSQFDQ- Ⅱ b07 |
07 |
7 |
11 |
11 |
|
|
WTSQFDQ- Ⅱ b08 |
08 |
8 |
12 |
12 |
|
|
WTSQFDQ- Ⅱ b09 |
09 |
9 |
13 |
13 |
|
|
WTSQFDQ- Ⅱ b10 |
10 |
10 |
14 |
14 |
|
|
WTSQFDQ- Ⅱ b12 |
12 |
12 |
16 |
15 |
|
|
WTSQFDQ- Ⅱ b14 |
14 |
14 |
18 |
17 |
|
|
WTSQFDQ- Ⅱ b16 |
16 |
16 |
22 |
20 |
|
|
WTSQFDQ- Ⅱ b18 |
18 |
18 |
24 |
22 |
|
|
WTSQFDQ- Ⅱ b20 |
20 |
20 |
26 |
24 |
Membranous AsymmetricVentricular Septal Defect (VSD)Occluder for Multi-Fenestrated Defects
|
Catalogue No |
Device Size |
A Waist Diameter (mm) |
B LV Disc Diameter (mm) |
C RV Disc Diameter (mm) |
H Waist Length (mm) |
|
SQFDQ- Ⅲ 04 |
04 |
4 |
12 |
8 |
|
|
SQFDQ- Ⅲ 05 |
05 |
5 |
13 |
9 |
|
|
SQFDQ- Ⅲ 06 |
06 |
6 |
14 |
10 |
|
|
SQFDQ- Ⅲ 07 |
07 |
7 |
15 |
11 |
|
|
SQFDQ- Ⅲ 08 |
08 |
8 |
16 |
12 |
|
|
SQFDQ- Ⅲ 09 |
09 |
9 |
17 |
13 |
|
|
SQFDQ- Ⅲ 10 |
10 |
10 |
18 |
14 |
|
|
SQFDQ- Ⅲ 12 |
12 |
12 |
20 |
16 |
|
|
SQFDQ- Ⅲ 14 |
14 |
14 |
22 |
18 |
|
|
SQFDQ- Ⅲ 16 |
16 |
16 |
24 |
20 |
|
|
SQFDQ- Ⅲ 18 |
18 |
18 |
26 |
22 |
Hubless Membranous AsymmetricVentricular Septal Defect (VSD)Occluder for Multi-Fenestrated Defects
|
Catalogue No |
Device Size |
A Waist Diameter (mm) |
B LV Disc Diameter (mm) |
C RV Disc Diameter (mm) |
H Waist Length (mm) |
|
WTSQFDQ- Ⅲ 04 |
04 |
4 |
12 |
8 |
|
|
WTSQFDQ- Ⅲ 05 |
05 |
5 |
13 |
9 |
|
|
WTSQFDQ- Ⅲ 06 |
06 |
6 |
14 |
10 |
|
|
WTSQFDQ- Ⅲ 07 |
07 |
7 |
15 |
11 |
|
|
WTSQFDQ- Ⅲ 08 |
08 |
8 |
16 |
12 |
|
|
WTSQFDQ- Ⅲ 09 |
09 |
9 |
17 |
13 |
|
|
WTSQFDQ- Ⅲ 10 |
10 |
10 |
18 |
14 |
|
|
WTSQFDQ- Ⅲ 12 |
12 |
12 |
20 |
16 |
|
|
WTSQFDQ- Ⅲ 14 |
14 |
14 |
22 |
18 |
|
|
WTSQFDQ- Ⅲ 16 |
16 |
16 |
24 |
20 |
|
|
WTSQFDQ- Ⅲ 18 |
18 |
18 |
26 |
22 |
Zero Rim EccentricVentricular Septal Defect (VSD) Occluderfor the Defect Close to Aortic Valve
|
Catalogue No |
Device Size |
A Waist Diameter (mm) |
B LV Disc Diameter (mm) |
C RV Disc Diameter (mm) |
H Waist Length (mm) |
|
SQFDQ- Ⅳ04 |
04 |
4 |
9 |
8 |
|
|
SQFDQ- Ⅳ 05 |
05 |
5 |
10 |
9 |
|
|
SQFDQ- Ⅳ 06 |
06 |
6 |
11 |
10 |
|
|
SQFDQ- Ⅳ 07 |
07 |
7 |
12 |
11 |
|
|
SQFDQ- Ⅳ 08 |
08 |
8 |
13 |
12 |
|
|
SQFDQ- Ⅳ 09 |
09 |
9 |
14 |
13 |
|
|
SQFDQ- Ⅳ 10 |
10 |
10 |
17 |
15 |
|
|
SQFDQ- Ⅳ 12 |
12 |
12 |
20 |
18 |
|
|
SQFDQ- Ⅳ 14 |
14 |
14 |
22 |
20 |
|
|
SQFDQ- Ⅳ 16 |
16 |
16 |
24 |
22 |
To build LepuMedical into a large-scale global company in the cardiovascular industry that provides products and solutions covering prevention, treatment and rehabilitation for the patients with cardiovascular and related diseases.
If you want to know more kinds of medical devices china, please visit our website.
Send product request
Other supplier products
| Equipments | Lepu Medical Technology ( Beijing ) Co., Ltd was established in 1999. As a professional lepu medical technologygroup, it is specialized in developi... | |
| Eel Cath™ Electrophysiology Diagnostic Catheter | Eel Cath™ Electrophysiology Diagnostic Catheter Eel Cath™ ep diagnostic cathetersare used for temporary intra-cardiac sensing, recor... | |
| PC ECG-500 ECG Workstation | PC ECG-500 ECG Workstation Details Product Features • Can be used in hospital, clinique, physical examination center, community health cente... | |
| LFR60 Infrared Forehead Thermometer | LFR60B handheld forehead thermometeris a non-contact digital infrared thermometer for fever. It is suitable for different users, such as babies, ch... | |
| Guide Wires | To develop LepuMedical into one of the largest cardiovascular products manufacturersand on the platforms of medical device, medicine, healthcare pr... |
Same products
| Full-Body Garment Stress Tester,FZ/T 70015 | Seller: Standard International Group (HK) Limited | TheFull-Body Garment Stress Testeris a precision instrument designed to quantify the pressure exe... | |
| Industrial Dry Cleaning Machine,industrial garment steamer | Seller: Standard International Group (HK) Limited | TheIndustrial Dry Cleaning and Energy-Saving Dryer Machinesare designed for high-performance, rel... | |
| MST Medical Compression Stocking Tester,compression sock tester | Seller: Standard International Group (HK) Limited | The MST Medical Compression Stocking Tester is a professional instrument designed to evaluate the... | |
| MMT Liquid Water Separation Tester,MMT Liquid Water Analysis Tool | Seller: Standard International Group (HK) Limited | Applications Textile Manufacturing:Testing waterproof fabrics for outdoor gear R&D:Developi... | |
| Dental material color stability tester | Seller: Standard International Group (HK) Limited | Application The applications of color stability testers for dental materials are mainly reflecte... |