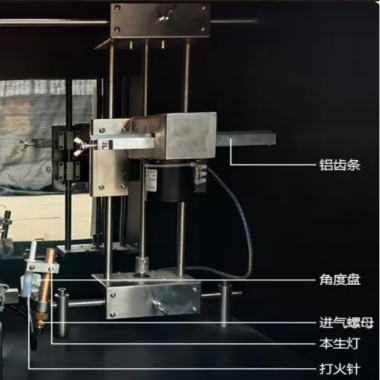
Infusion device Leakage negative pressure tester
The Infusion Device Leakage Negative Pressure Tester is designed to evaluate the airtightness of syringes, infusion sets, and related medical devices under negative pressure. The instrument applies precise vacuum conditions to detect leaks, ensuring the integrity of medical devices for patient safety. It is suitable for laboratory testing, production quality control, research and development, and regulatory certification.
Application
The tester is suitable for leakage testing of medical infusion devices in the following industries and products:
(1) Medical Quality Control: Ensures syringes, infusion sets, and other injection devices meet strict leakage standards to protect patient safety.
(2) Pharmaceutical Manufacturing: Validates packaging and device integrity for sterile medical products.
(3) Research & Development: Supports design validation, accelerated aging, and product optimization of infusion devices.
(4) Certification & Regulatory Testing: Enables compliance with national and international medical device standards and regulatory requirements.
(5) Third-Party Testing Laboratories: Provides standardized, repeatable leakage testing for medical device certification.
Standards
The instrument is compliant with the following standards, including relevant international regulations:
(1) GB 8368-2005 – Disposable Infusion Sets for Single Use — Gravity Infusion Type, Airtightness Testing Requirements
(2) ISO 8536-4 – Sterile Syringes for Medical Use — Leakage Test Methods
(3) ISO 13485:2016 – Medical Devices — Quality Management Systems for Regulatory Compliance
(4) ASTM F2392-04 – Guide for Leak Testing of Medical Fluid Delivery Devices
Features
(1) Precision negative pressure testing: -10 to -30 kPa with ±0.5 kPa accuracy
(2) Dual-temperature testing: 23 ±1°C and 40 ±1°C to simulate real-world and accelerated aging conditions
(3) PLC-driven automated control: ensures repeatable and reliable results
(4) Safety interlocks and auto-shutoff prevent device damage
(5) Compact footprint (500×600×500 mm) suitable for labs and production lines
(6) Adjustable test time (1–16 seconds) with intuitive digital touchscreen interface
(7) Distilled water flushing system in vacuum chamber eliminates air bubbles for accurate detection
(8) Stainless steel construction for corrosion resistance and easy cleaning
Technical Parameters
| Parameter | Specification |
|---|---|
| Control Mode | Automatic (PLC) |
| Test Pressure Range | -10 kPa to -30 kPa (Adjustable) |
| Pressure Accuracy | ±0.5 kPa |
| Temperature Range | 23 ±1°C / 40 ±1°C |
| Test Time | 1–16 seconds (Adjustable) |
| Chamber Material | Medical-grade stainless steel |
| Power Supply | AC 220V ±10%, 50Hz |
| Dimensions (L×W×H) | 500 mm × 600 mm × 500 mm |
| Net Weight | 30 kg |
| Display | LED Touchscreen (Pressure/Time/Status) |
Accessories
(1) Vacuum chamber with integrated distilled water flushing system
(2) Standard connectors for syringes and infusion sets
(3) Power cable
(4) Operation manual
(5) Calibration certificate
Test Procedures
(1) Install the infusion device or syringe in the vacuum chamber connector
(2) Seal the chamber properly and ensure the distilled water flushing system is operational
(3) Set the test pressure (-10 to -30 kPa) and test time (1–16 s) on the touchscreen interface
(4) Select the desired temperature condition (23°C or 40°C)
(5) Start the automated test
(6) Monitor the pressure decay and leakage rate in real time on the display
(7) Review results and mark the device as “Qualified” or “Unqualified”
(8) Release the pressure and remove the tested device
(9) Repeat for additional samples
Maintenance Information
(1) Clean the vacuum chamber and connectors after each test to remove residues
(2) Inspect seals, connectors, and chamber surfaces for wear or damage
(3) Calibrate pressure sensors and temperature control systems regularly
(4) Store the instrument in a clean, dry, and ventilated environment
(5) Avoid excessive force or impact on the instrument to prevent damage
(6) Maintain logs for calibration, testing, and maintenance to comply with regulatory requirements
在线联系供应商
Other supplier products
| Transmission belt flammability and flame diffusion characteristics tester | Product Introduction The conveyor belt flammability and flame diffusion tester is suitable for testing the flammability and flame propagation char... | |
| Shoe heat insulation testing machine -- sand bath | Standard GB/T 20991: Determines thermal insulation of safety shoe soles under simulated hot surface exposure. EN ISO 20344: European standard for... | |
| Melt Index Tester | Standards: ISO 1133. ASTM D1238. GB/T 3682. JIS K7210 Main uses: The melt flow rate meter uses automatic sampling and balance weighing to determ... | |
| Metal Bone Screws Self - tapping Tester | Standard ISO 6475: Implants for surgery – Wear of total hip-joint prostheses ASTM F543: Standard Specification and Test Methods for Metalli... | |
| UV Curing Machine | Description UV is the ultraviolet (Ultra-VioletRay) English abbreviation. Industrial UV wavelength to 200nm to 450nm for its scope of applicatio... |
Same products
| Full-Body Garment Stress Tester,FZ/T 70015 | 卖方: Standard International Group (HK) Limited | TheFull-Body Garment Stress Testeris a precision instrument designed to quantify the pressure exe... | |
| Industrial Dry Cleaning Machine,industrial garment steamer | 卖方: Standard International Group (HK) Limited | TheIndustrial Dry Cleaning and Energy-Saving Dryer Machinesare designed for high-performance, rel... | |
| MST Medical Compression Stocking Tester,compression sock tester | 卖方: Standard International Group (HK) Limited | The MST Medical Compression Stocking Tester is a professional instrument designed to evaluate the... | |
| MMT Liquid Water Separation Tester,MMT Liquid Water Analysis Tool | 卖方: Standard International Group (HK) Limited | Applications Textile Manufacturing:Testing waterproof fabrics for outdoor gear R&D:Developi... | |
| Dental material color stability tester | 卖方: Standard International Group (HK) Limited | Application The applications of color stability testers for dental materials are mainly reflecte... |