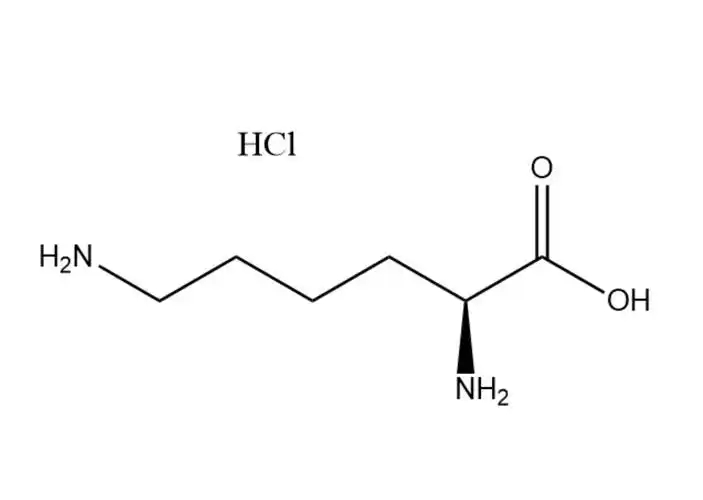
Calcium carbonate
Calcium carbonate has three different crystal structures:
Calcite: It is the most common structural form, belonging to the trigonal crystal system, and has high symmetry and stability. The crystal forms of calcite are diverse, with common ones including rhombohedrons and trigonal hedrons. In nature, marble, limestone and other materials are mainly composed of calcite.
Aragonite, also known as aragonite, belongs to the orthorhombic crystal system. Its crystal structure and physical properties are different from those of calcite. The stability of aragonite is relatively low and it can be transformed into calcite under certain conditions.
Aragonite: It is a metastable structural form and belongs to the hexagonal crystal system. Aragonite is relatively rare in nature and usually forms under specific physical and chemical conditions, such as in living organisms or during certain rapid precipitation processes.
Другие товары поставщика
|
|
Magnesium Gluconate |
Applications of Magnesium Gluconate 1. Nutritional Fortification in Food and Beverages (1)Magnesium Supplementation: As a bioavailable magnesium so... |
|
|
Magnesium Citrate |
Magnesium citrate, a highly bioavailable form of magnesium combined with citric acid, plays a significant role in nutrition and healthcare due to i... |
|
|
Monosodium fumarate |
Monosodium fumarate, a sodium salt of fumaric acid, is primarily valued for its functional properties in various industries, with its most prominen... |
|
|
Magnesium Lactate Powder |
Magnesium lactate in Industrial and Technical Applications Chemical Synthesis: Used as a magnesium source in laboratories for synthesizing other ma... |
|
|
Calcium Carbonate |
The main uses of Calcium CarbonateI. Industrial Field· Building materials: They are important raw materials for manufacturing cement, lime, ... |
Все товары поставщика
Похожие товары