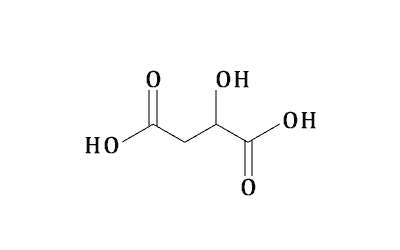
Magnesium Citrate


The physical and chemical properties of magnesium citrate can be summarized from the following aspects:
1. Physical properties
Appearance: Magnesium citrate is usually a white or yellowish powder, and sometimes it is crystalline or granular. Its appearance may vary depending on the production process and storage conditions.
· Solubility: Magnesium citrate has good solubility in water and can be dissolved into magnesium ions and citrate ions. However, the solubility in alcohol solvents is small. In addition, its solubility may also be affected by temperature, pH value and other factors.
· Stability: Magnesium citrate is relatively stable at room temperature and pressure and is not easy to decompose. However, in a humid environment, it may absorb moisture from the air and become damp. Therefore, it needs to be kept dry when storing.
Hygroscopicity: Magnesium citrate can absorb moisture in air, and when the relative humidity reaches a certain degree (such as 80%), it may lose part of the crystal water.
2. Chemical properties
· Reactivity: Magnesium citrate is a weak acid salt that can react with strong bases to produce citric acid and magnesium ions. This reactivity makes magnesium citrate potentially useful in a variety of chemical reactions.
Ionic: When dissolved in water, magnesium citrate will dissociate into magnesium ions and citrate ions. This ionic property makes magnesium citrate play an important role in electrolyte balance and biological metabolism.
· Acid-base: Magnesium citrate has a certain acid-base, but its specific acid-base depends on the concentration and pH of its solution. Under appropriate conditions, magnesium citrate can be used as a buffer to adjust the pH of the solution.
3. Preparation method
There are two main methods for the preparation of magnesium citrate: chemical synthesis and physical methods.
· Chemical synthesis: citric acid is reacted with magnesium oxide to produce magnesium citrate. The reaction equation is C6H8O7 + MgO → Mg(C6H5O7)2 + H2O. The magnesium citrate prepared by this method has high purity and stable quality.
· Physical method: By mixing citric acid and magnesium oxide powder evenly and heating to the appropriate temperature, it reacts to produce magnesium citrate. This method is simple to operate, but may be affected by reaction conditions and lead to product quality fluctuations.
Magnesium citrate has unique physical and chemical properties, and has wide application prospects in food, medicine, cosmetics and other fields. In the process of use, it is necessary to choose the appropriate magnesium citrate product according to the specific application scenario and requirements, and pay attention to the storage conditions to avoid moisture and deterioration.
Other supplier products
|
|
Sodium malate |
Sodium malate, derived from malic acid, is sometimes used in the food industry to enhance the taste of certain products.Malic acid itself is a natu... |
|
|
Ferric Sodium Edetate |
Ferric sodium edetate, (sodium ferric ethylenediaminetetraacetate) possesses several properties that make it suitable for various applications.Here... |
|
|
Ferric sodium edetate |
|
|
|
Ferric Phosphate |
Ferric phosphate, also known as iron(III) phosphate, is a chemical compound composed of iron and phosphate ions.It is represented by the chemical f... |
|
|
Chinese Exporter |
When transporting calcium lactate gluconate, it is essential to ensure the product's safety and stability. Here are some key transportation precaut... |
供应产品
Same products